Aggregation in the Pharma Industry: Product Traceability Beyond Boundaries
Pharmaceutical companies will achieve considerable cost-savings by using traceability and aggregation solutions. A large part of these savings come from improvements in process and supply chain efficiency. Many companies see this solution as a major competitive advantage in today’s global economy.
Download the white paper now and discover why aggregation pharma is the future-proof solution!
Aggregation Pharma – How Does It Work?
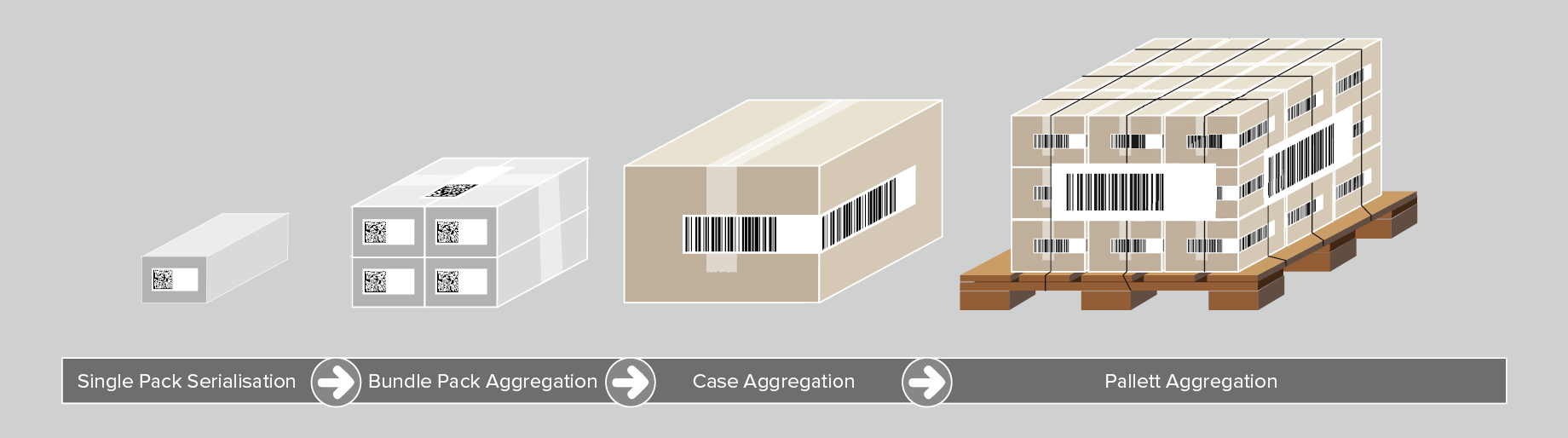
Aggregation enables companies to trace their products from the moment of production, according to the parent-child principle. Once a blister pack is placed into a folding box (child), its serialization code is scanned. In the next step, several folding boxes are packaged into a bundle (parent), and their serial numbers are linked to it. Several bundles can be also aggregated and related to a shipping box (the next higher-ranking parent level). This parent-child dataset is further linked to a pallet.
Aggregation of pharmaceutical products is a process, in which each participant in the supply chain takes over the information from the predecessor and additionally supplements it with its own data. This begins with the manufacturer, travels via the packaging to the third party logistics company (3PL), on to the wholesaler, etc. Each data aggregate is saved in a database before being passed on to the next participant in the supply chain.
White Paper: Aggregation in Pharma
The white paper answers the questions of tomorrow and provides information on aggregation, as a future-oriented technology. It illustrates the basic principles of this new technology and shows in detail how drug manufacturers can fully track their product, no matter where it is. At the same time, the white paper provides an insight into the improvement potential of all processes along the supply chain using the aggregation pharma approach.
Benefits of Aggregation for Pharmaceutical Companies
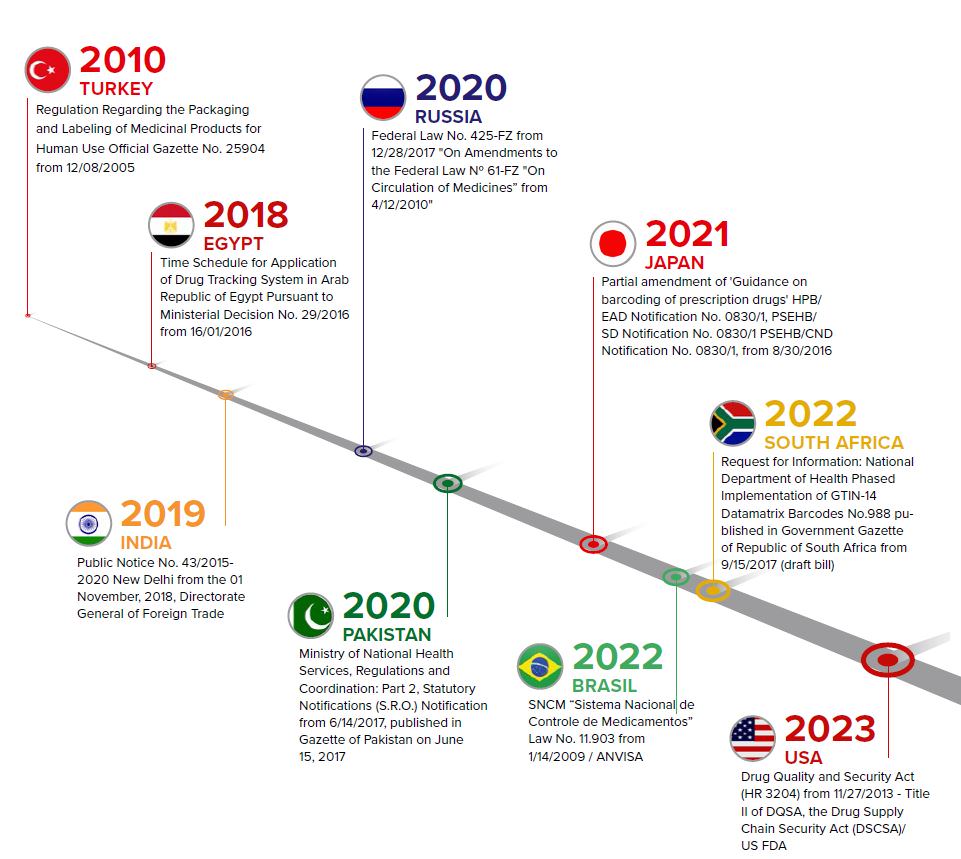
- Flexibility to meet Serialization & Aggregation requirements of multiple countries. Aggregation Pharma is required, or will be required, by different countries as a part of their national Serialization & Aggregation regulations (see the graph). Due to globalization, manufacturers can achieve long-term success only if they can quickly adapt to new export markets.
- Optimization of warehousing and supply chain activities. Aggregation simplifies reporting and verification of the product at one or more stops along the supply chain. By scanning the barcode, all the necessary data can be obtained in a matter of seconds, which leads to significant time and resource savings.
- Fast decommissioning of shipping cases and pallets. Shipment pallets could be decommissioned in bulk from the cloud system. In case the whole pallet or a part of it has been damaged during transportation, aggregation will significantly simplify booking out the damaged products from the system.
- Efficient product re-calls or returns. Aggregation Pharma technology facilitates the ability to manage the entire process of inventory returns better. It also helps manufacturers perform targeted recalls of specific product units instead of an extensive, unnecessary amount.
Aggregation Solutions from Wipotec
Open interfaces and easy operation
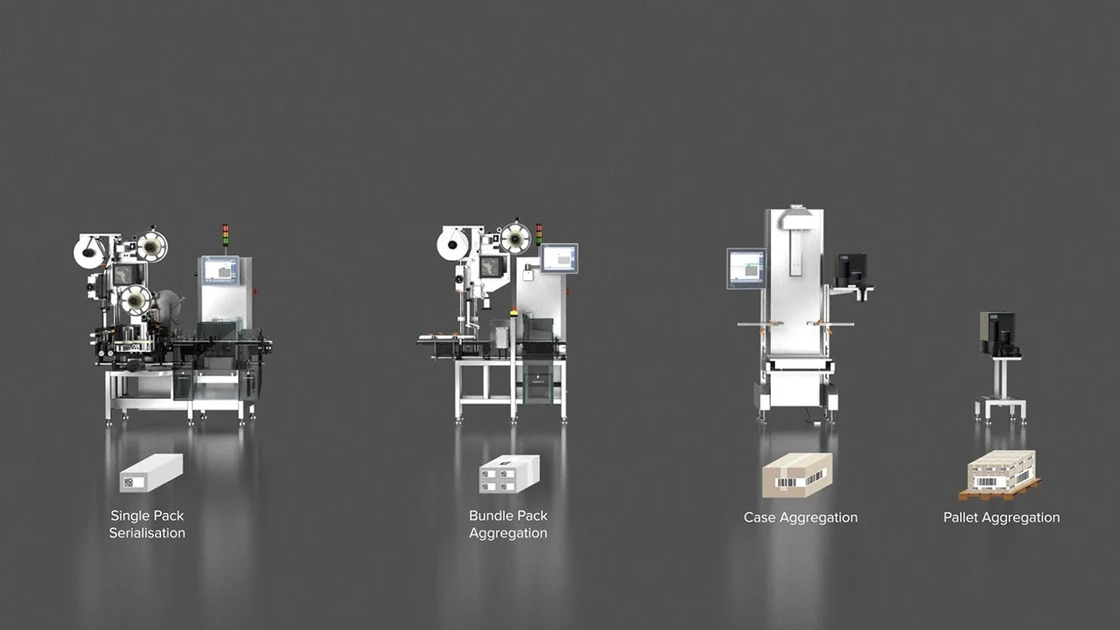
TQS Track & Trace System is a highly flexible, modular Serialization & Aggregation platform, that easily integrates into packaging lines. All steps in the serialization and aggregation process are recorded and the data is read and stored according to the "parent-child principle".
Looking for a fast plug-and-play solution?
TQS FAST TRACK – automated serialization and aggregation within 6 weeks.
Find your suitable Track & Trace solution
TQS-CP for the perfect semi-automatic aggregation of folding boxes - from a shipping case to a pallet
TQS-CP stands for semi-automatic aggregation of manually packed folding boxes. It lets you conveniently assign units from a lower level (folding box or bundle) to a higher packaging level (shipment carton) and a pallet.
Semiautomatic aggregation of shipping cases or pallets
Reliable 360° inspection for semi-automatic aggregation of bottles and vials
TQS-CP-Bottle is designed for perfect integration in bottle or vial production lines. The system can be set up directly at the outlet of any conventional labelling machine to verify the previously serialized labels on individual cylindrical containers via an omnidirectional, 360° inspection.
All-round inspection, verification, semiautomatic aggregation of pharmaceutical products
TQS-BP for simple aggregation of bundles – flexible and format-independent
TQS-BP is ideal for the simple implementation of the first aggregation level. The system combines the serial numbers from multiple single packages into one unique serial number for the bundle.
Aggregation of single packages into bundles (1st level of aggregation)
Application Report
Batch coding, serialization and aggregation at Klocke
Find out how the Klocke Group applies advanced technology to achieve highest quality standards. Dominik Katarzynski, Managing Director of Klocke Packaging-Service in Weingarten, Germany, shares his strategic approach and experience in addressing their challenges:
- complex regulations for serialization and aggregation
- specific customer requirements
- frequent format changeovers
The packaging lines are equipped with various Wipotec solutions from checkweighing to batch coding, serialization and Tamper-Evident labeling all the way to the aggregation of bundles and cases - or as he puts it in the interview:
“We have now installed the 50th Wipotec unit – I think that says it all.”